How Did Dalton Describe the Atom
He defined an atom to be a ball-like structure as the concepts of atomic nucleus and electrons were unknown at the time. Daltons atomic theory also stated that all compounds were composed of combinations of these atoms in defined ratios.

John Dalton Biography Discoveries Atomic Model Facts Britannica
There were of course no scientific instruments at that time but he reasoned philosophically that there had to be a smallest unit of matter which he called the atom.

. Based on all his observations Dalton proposed his model of an atom. The first part of his theory states that all matter is made of atoms which are indivisible. This remained true until 1803 when an English chemist John Dalton came up with actual experimental evidence for the existence of atoms.
All atoms of an element are identical. While all atoms of an element were identical different elements had atoms of. John Dalton was an English chemist in the late 18th century who theorized that each element had its own kind of atom and that these could combine to form compounds but he couldnt explain.
Different kinds of atoms chemically combine to form elements. Atoms are made up of combinations of elements. It is often referred to as the billiard ball model.
Everything is composed of atoms which are indivisible building blocks of matter and cannot be destroyed. The History of the Atomic Model. Daltons atomic theory proposed that all matter was composed of atoms indivisible and indestructible building blocks.
The atom cannot be created or destroyed. The Five Main Points of Daltons Atomic Theory. The main points of Daltons atomic theory are.
Elements are made up of atoms arranged in whole-number ratios. His argument that each element had its own kind of atom was counterintuitive to those who believed that having so many different fundamental particles would destroy the simplicity of nature but Dalton. How did Dalton describe the relationship between atoms and elements.
The word atom comes from the Greek word that means. Atoms of a given element are identical in size mass and other properties. Elements are made of extremely small particles called atoms.
They formulated the key concepts of the law of conservation of mass and the existence of atoms as the building blocks of all matter using their knowledge of chemical reactions. Daltons atomic theory proposed that all matter was composed of atoms indivisible and indestructible building blocks. It stated that all matter was made up of small indivisible particles known as atoms.
If you asked Dalton to draw the diagram of an atom he wouldve simply drawn a circle. Dalton claimed that atoms of different elements vary in size and mass and indeed this claim is the cardinal feature of his atomic theory. Daltons atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.
The modern Atomic Model was first developed by two key scientists Lavoisier and Dalton with the help of others. While all atoms of an element were identical different elements had atoms of differing size and mass. According to Dalton what happens to atoms during a chemical reaction.
Daltons atomic theory was a scientific theory on the nature of matter put forward by the English physicist and chemist John Dalton in the year 1808. Daltons Atomic Theory the laws its built on and the law he derived from it. An element is made up of one kind of atom.
Atoms cannot be subdivided created or destroyed. He also mentioned that atoms of different elements can combine to form. John Dalton described an atom to be the smallest particle that is inside an element.
How did Dalton describe the relationship between atoms and elements. Dalton based his theory on the law of conservation of mass and the law of constant composition. How did John Dalton discover atom.
The splitting of the atom in the 20th century could most likely not have been accomplished without Dalton laying the foundation of knowledge about the atomic makeup of simple and complex molecules. Atoms of different elements differ in size mass and other properties. All substances according to Daltons atomic theory are made up of atoms which are indivisible and indestructible building units.
Learn with flashcards games and more for free.

Dalton S Atomic Theory Postulates Limitations Concepts Videos Q A

Dalton S Atomic Theory Youtube

John Dalton His Theory Of The Atom Dalton S Model Of The Atom Ppt Download

What Is John Dalton S Atomic Model Universe Today

Dalton S Atomic Theory Atomic Theory Atom Model John Dalton Atomic Theory
4 3 Dalton S Atomic Theory Chemistrysaanguyen
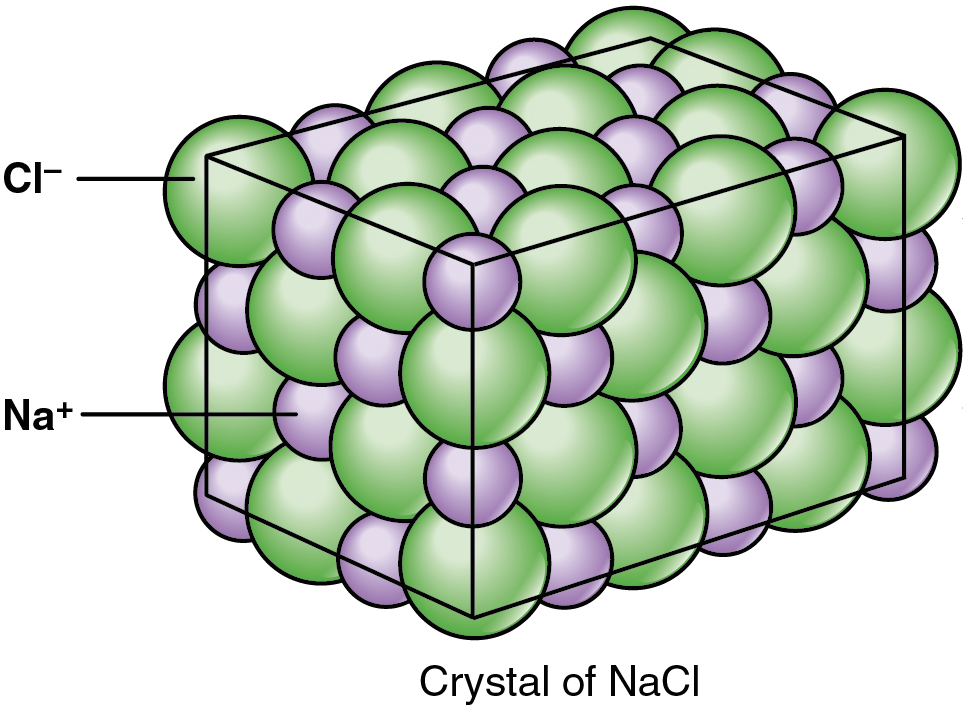
Dalton S Atomic Theory Article Khan Academy

Scientific Explorer May 2012 Dalton Atomic Model Plum Pudding Model Atom Model

What Is John Dalton S Atomic Model Universe Today
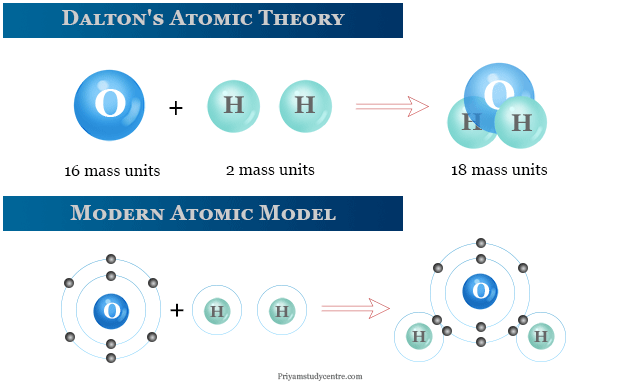
Dalton S Atomic Theory Postulates Definition Model

Dalton S Atomic Theory Updated 2021 2022 Coolgyan
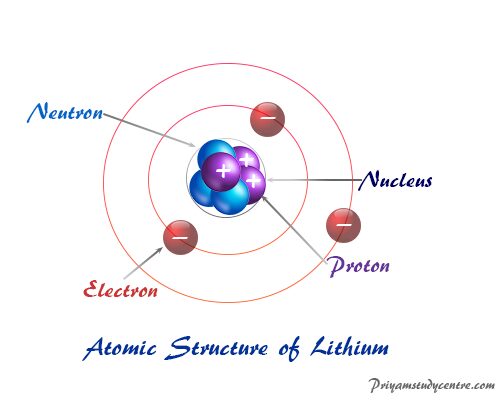
Dalton S Atomic Theory Postulates Definition Model

John Dalton Atomic Theory Discovery Experiments Biography

John Daltons Atom Theory Model Did Include The Charges Dalton Atomic Model Atomic Theory Atom Model

Daltons Atomic Theory And Its Modifications
Atomic Models John Dalton Thomson S Plum Pudding Rutherford Bohr Explained With Distinctions

John Dalton Atomic Theory Discovery History Example Chemistry Qna

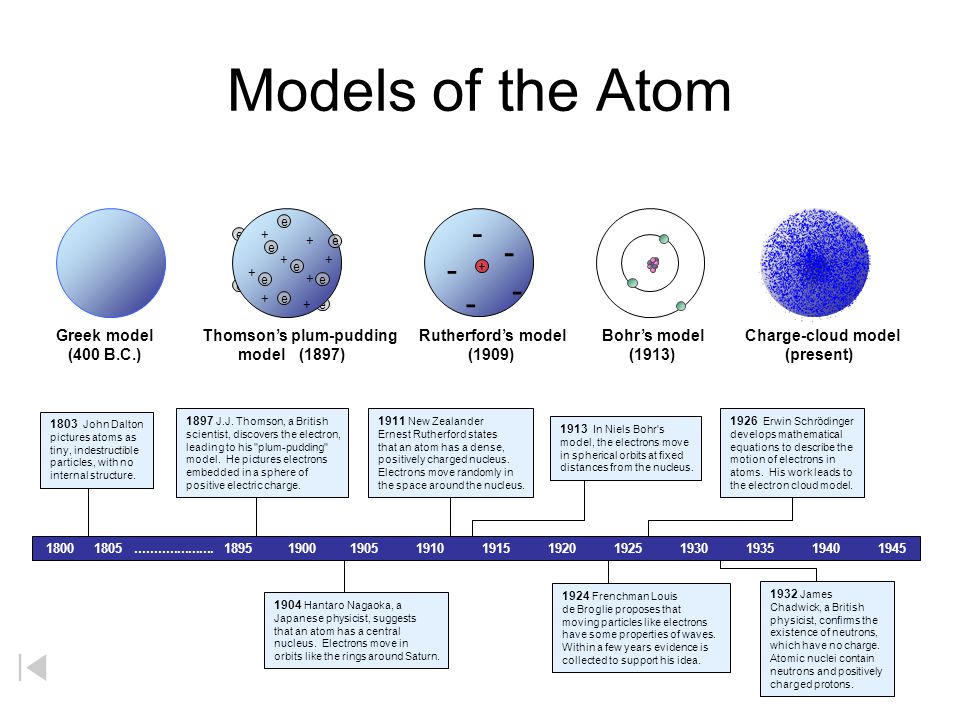
Comments
Post a Comment